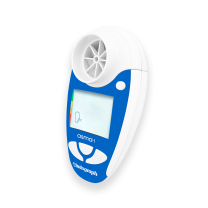
Vitalograph copd-6 Screener
Fast & accurate COPD screener to identify those at risk of COPD at the pre-symptomatic stage.
The Vitalograph copd-6™ identifies those at risk of COPD at the pre-symptomatic stage. The device screens out those whose FEV1 is normal.
The copd-6 screener allows early medical intervention and facilitates better clinical outcomes.
- Monitors COPD patients using their ‘number’, the obstructive index which is based on a percent of predicted.
- Facilitates ‘case selection’ so that spirometry can be focused on those likely to be diagnosed with COPD.
- Screens out anyone with normal FEV1 without the risk of false COPD negatives.
- Displays FEV1, FEV6, ratio and % predicted, obstructive index, COPD classification and lung age.
- Displays the GOLD COPD classification (stage I – IV) to help recognize the need for a change in the patient’s management plan.
- Built-in quality of blow indicator.
- Large, easy-to-read display
One of our 4000 Series of respiratory monitors and screeners, the COPD-6 comes with:
- Carry pouch
- Instructions for use
- 2 x AAA Batteries
Product Code: 40201
Respiratory Monitor copd-6
Model 4000
Dimensions 109mm (length) x 63mm (width) x 42mm (height)
Flow Detection Principal Stator rotor
Accuracy Better than ± 3%
Back Pressure Less than 0.15kPa/L/second @ 14L/s
Measurement Range 0 – 9.99 L BTPS
Power Supply 3V (2 x 1.5V AAA batteries)
Battery Life 3 months of use, 3 tests per day
Quality Check Slow start of test (Vext>5%) or a cough detected in the first second
Performance Standards & Guidelines ATS/ERS 2019, ISO 23747:2015, ISO 26782:2009
Auto Power Down Time 2 minutes
- 28501 Eco BVF (100)
- 28572 Eco BVF + Disposable Noseclips (80)
- 20242 SafeTway Mouthpieces (200)
- 20303 Disposable Noseclips (200)
- 20980 SafeTway Mouthpieces mini (50)
- 20991 SafeTway Mouthpieces long (130)
- 40167 Pouch Spare (x10)