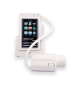
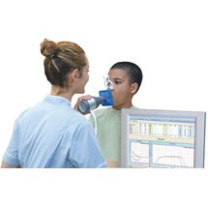
In2itive Spirometer with Spirotrac Software
Lightweight handheld spirometer providing portability with PC and network connectivity through Spirotrac® Software. For adult and pediatric testing, with data storage for over 10,000 subjects.
Supplied with powerful Spirotrac Software, enabling the ability to add further testing options and easy data synchronization to PCs, laptops, and networks. Generate detailed PDF reports for filing and uploading to electronic medical record (EMR) systems.
A color touch screen display and an intuitive icon driven menu, makes the In2itive quick and simple to operate.
Simple, validated hygiene system using Bacterial Viral Filters (BVFTM) means that keeping the device clean is easy and inexpensive.
The In2itive comes with:
- Vitalograph Spirotrac Software
- Cradle
- USB cable
- Charger plug
- Stylus
- Vitalograph Reports Software
Features:
- VC, FVC, and bronchodilator responsiveness testing. Over 50 available parameters, GLI predicted equations and Z-scores.
- Capability to interface with EMR systems via Spirotrac software
- Low running costs. No need for costly disposable sensors, turbines, or flow tubes.
- Save time & money on cleaning by using Vitalograph Bacterial Viral Filters (BVFTM).
- Real-time curves and incentive animations encourage optimal performance.
- Remote flowhead enables the subject to view incentives while performing the test.
- Easy data synchronization with Spirotrac Software. No need to re-enter subject details on the device.
- Cradle for battery charging, connection to a PC and automatic data exchange.
- Be sure of accurate test results through quick and easy calibration verification routines as recommended by international spirometry guidelines (ATS/ERS).
- Electrical outlet or rechargeable battery powered for total flexibility and portability.
Comes with 5-Year Warranty with unlimited technical support if registered within 30-days of purchase.
| Test types: | Single breath tests, flow/volume loops, multi-breath testing, tidal breathing and combined VC/FVC type test methods supported |
|---|---|
| Selectable Parameters: | VC; IVC; IC; VT (TV); TLC; RV; IRV; ERV; FRC; FVC; FIVC; FEV1; FEV3; FEV6; FVC; FEV1/VC; FEV1/FVC; FEV3/VC; FEV3/FVC; FEV1/FEV6; FEF75; FEF50; FEF25; FEF25-75; FEF25-75/FVC; FIV1; PIF, FIV1/ FIVC, FIF25, FIF50, FIF75, FEF50/FIF50, FET, MVVind, FEV1 Ratio; FEV0.5; PEF L/min; PEF L/s; FEF 0.2-1.2; FEF 75-85%; FEF25%; FEF50%; FEF75%; FMFT; FIF25%; FIF50%; FIF75%; FEV0.75; Lung Age. |
| Predicted Values Selectable: | GLI, ERS/ECCS, NHANES, Polgar, Pereira, Berglund,Forsche, Gutierrez,Hedenström, Taiwan;Knudson, Crapo; Hsu; KNLW; Viljanen; SEPAR; Gulsvik; SBPT; Tamura; Ip; Quanjer; Wang; Dockery and more |
| Flow Technology: | Fleisch Pneumotachograph |
| Resolution: | 10 mL volume; 0.01 L/s flow |
| Data Storage: | Stores up to 10,000 subjects and hundreds of test sessions |
| Accuracy when in Operating Range: | Volumes: Better than ± 2.5% (Max 10L / Min 0L) Flows: Better than ± 10% (Max 16L/s / Min 0.02L/s) Linearity: ± 5% in range 0.1 L/s to 16 L/s |
| Dimensions Device: | 160mm x 100mm x 45mm (with flowhead attached) |
| Net Weight: | Device: 230g |
| Recommended Operating Temperature Range: | 17 – 37°C; Design Limits 10-40°C |
| Connectivity: | USB 2 |
| Performance Standards & Guidelines: | ATS/ERS 2019, ISO 23747:2015 & ISO 26782:2009 |
| Safety Standards: | EN 60601-1:2006 |
| Display | Color touch screen |
- 70201 Spirotrac Multi-User Licence
- 28750 BVF_ Bacterial/Viral Filters (50)
- 20242 SafeTway Mouthpieces (200)
- 20201 Cardboard Mouthpieces (200)
- 20303 Noseclip Disposable (200)
- 36020 Precision Syringe 3-L
- 66149 Thermal printer paper (5)
- 42084 Flow conditioning meshes (10)